Autoimmune thyroiditis, lymphocytic thyroiditis, Hashimoto’s thyroiditis, or Hashimoto’s disease, is an autoimmune disorder characterized by inflammation of the thyroid gland as a result of an autoimmune reaction to T lymphocytes (T cells). The name “Hashimoto” originated from the Japanese doctor, Hakaru Hashimoto, who first depicted the disease in 1912. Hashimoto’s Disease has become more and more prevalent in recent years, primarily affecting women; of every 100,000 people, 2.2 men and 498.4 women are affected per year worldwide.
Contenido
Signs and symptoms of Hashimoto’s disease
It can be difficult to detect the signs and symptoms of Hashimoto’s disease at first, as the disease typically developes slowly. As the disease progresses, damage to the thyroid results in a drop in thyroid hormone levels in the blood.
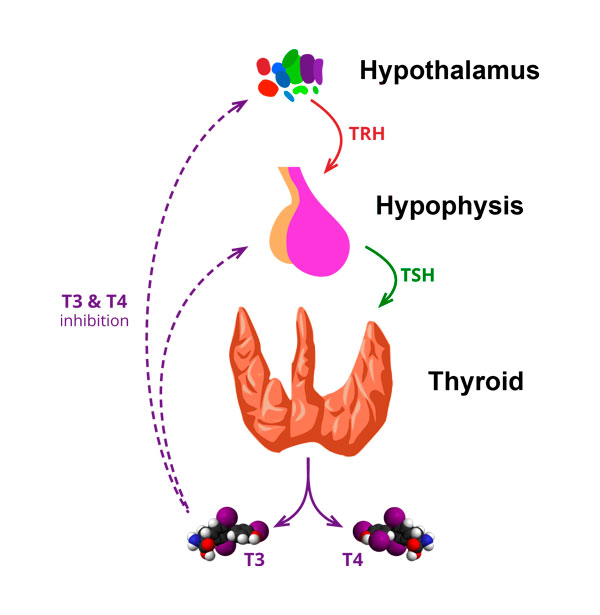
The thyroid hormones T3 and T4 fulfill numerous vital functions. They regulate metabolism, body temperature and cardiac output. They play a role in brain development, bone metabolism, muscle function, and bowel movements.
At the onset of Hashimoto’s disease, T3 and T4 concentrations are at normal levels; metabolic condition is unaffected and the patient appears to be in a healthy, euthyroid state. As the thyroid gland weakens, however, concentrations of TSH – the hormone that stimulates T3 and T4 secretion – begin to increase. The thyroid reserve and T4 levels begin to decrease. Though T3 levels are able to hold stable initially, they eventually begin dropping as well. As the disease advances, lymphocytic infiltration and fibrosis bring about a continual decrease in thyroid efficiency.
The signs and symptoms of Hashimoto’s disease are similar to those of an underactive thyroid gland, also known as hypothyroidism. In the case of Hypothyroidism, there are a number of rather nonspecific symptoms which begin to appear. Among them are: fatigue, lethargy, constipation, intolerance to cold, stiffness, muscle cramps, carpal tunnel syndrome, loss of appetite, weight gain, dry hair, hair loss, and dry or pale skin. In the most serious cases, central nervous system and kidney damage can occur, sometimes even resulting in the patient going into a coma.
Onset of Hashimoto’s disease
The origin of Hashimoto’s disease has been widely studied in recent years, due to its increasing incidence.
Three agents have been found to be implicated in the development of Hashimoto’s disease:
- A (genetically) altered immune system
- A stimulus (antigen) which induces an immune system response
- A permeable intestinal barrier which allows antigens to enter into the bloodstream
A genetically altered immune system
Certain genes have been identified as susceptible to the development of Hashimoto’s disease. Some of these genes, HLA DR3, DR5, DQ7, CTLA-4, and PTPN22 for example, have been linked to other autoimmune diseases as well. At present, the only thyroid-specific gene that demonstrates an association with Hashimoto’s disease is the thyrogobulin (TG) gene.
A stimulus (antigen) which induces an immune system response
An antigen is any substance that causes your immune system to produce antibodies against it. This means your immune system does not recognize the substance, and is trying to fight it off. An antigen may be a substance from the environment (foreign antigen) such as chemicals, bacteria, viruses, certain proteins in food, or pollen. An antigen may also form inside the body (autoantigen).
A permeable intestinal barrier which allows antigens to enter into the bloodstream
In normal functioning our intestinal wall acts as a selective barrier that allows nutrients to pass through while meanwhile blocking the passage of potentially harmful substances. Physical, biochemical and immunological components coordinate with each other to create this defensive wall. For those suffering from Hashimoto’s disease, an alteration in the permeability of the intestinal wall triggers an autoimmune process. Having undergone surgery or stress, received burns, suffered trauma, sepsis, pancreatitis, intestinal infections or inflammation all increase one’s susceptibility to the disease; as does intolerance to certain foods (cow’s milk), bacterial overgrowth, ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) or alcohol consumption.
Other factors that are implicated in Hashimoto’s disease are:
- Infections: Certain microorganisms have been identified as triggers for the onset of autoimmune diseases due to their molecular similarity with thyroid structures, along with the fact that they create super-antigens which can result in an immune reaction affecting the thyroid gland. The identified microorganisms are: Epstein Barr virus (EBV), Cytomegalovirus (CMV), Yersinia enterocolitica, Borrelia burgdorferi, Candida albicans, Toxoplasma gondii, herpes simplex virus, hepatitis C virus and parvovirus B19.
- Poisoning by toxins and heavy metals: Autoimmune thyroiditis can also be triggered by toxic substances via their interaction with the immune system. These substances can damage both the intestinal mucosa and the thyroid cells. Exposure to polychlorinated biphenyl (PCB), dioxin, and heavy metals like mercury have been linked to high levels of antithyroglobulin and anti-peroxidase antibodies. Similarly, exposure to phthalates have been linked to the exacerbation of thyroid disease. Too, elevated levels of bisphenol A have been connected to increased levels of circulating thyroid hormones.
- Vitamin D deficiency: A significant deficiency of vitamin D has been observed among patients suffering from Hashimoto’s disease. This deficit contributes to a reduced tolerance to one’s own antigens which stimulates the production of auto-antibodies and contributes to direct cell damage, ultimately leading to autoimmune related conditions.
- Gluten intolerance: those with Hashimoto’s disease are more likely to suffer from celiac disease. This may be due to their increased immune sensitivity, to a deficiency of elements such as iodine and selenium (necessary for the synthesis of thyroid hormones) as a result of intestinal malabsorption associated with celiac disease, and/or to antibodies that target both intestinal and thyroid tissues. Gluten can generate intestinal permeability by directly damaging the intestinal mucosa in non-celiac patients as well.
- Excess iodine: Iodine is essential for the production of thyroid hormones. Poor iodine intake is associated with low thyroid-hormone production. Excess iodine can also have adverse effects. While the underlying mechanism has not yet been adequately substantiated, it appears that the immunological response to thyroglobulin is greater, and that iodine has a direct toxic effect on thyroid cells. To prevent this damage, it is vital that adequate amounts of selenium are maintained, in this way preserving an appropriate selenium-iodine ratio.
- Hormonal factors: The difference in the frequency of the disease and in the levels of autoantibodies among men and women, as well as the relationship between the disease’s progression and pregnancy could be explained by a series of hormonal factors. Estrogen, for example, is able to suppress diseases mediated by one type of lymphocyte (Th1 T lymphocytes) while at the same time potentiate diseases mediated by others (Th2 T lymphocytes).
- Stress: there is increasing evidence of the negative effect that chronic stress has on our health. With regard to Hashimoto’s disease, there are studies suggesting that stress hormones can lead to an increased immune response in the thyroid gland. Other studies show that reducing the immunosuppressive effect of hormones that occurs in the wake of a stressful event can trigger an autoimmune rebound reaction that can result in thyroid disease. The increase in cortisol during chronic stress also inhibits the production of TRH, the hormone responsible for the stimulation of thyroid hormone synthesis.
Treatment of Hashimoto’s disease: conventional vs multidisciplinary
Conventional treatment for Hashimoto’s disease includes administering a synthetic T4 thyroid hormone, monitoring the patient, and making subsequent dosage adjustments. Other measures, such as a change in diet, physical activity and stress management have demonstrated a capacity to slow the progression of the disease and greatly improve the quality of life of those who suffer from it.
While the use of synthetic T4 hormone in conventional treatment is able to control hypothyroidism in the majority of patients, a certain percentage, despite following dosage recommendations, remain symptomatic.
In addition, up to 15% of patients with TSH levels within the normal range have below-average T3 levels. Some patients taking Levothyroxine (synthetic T4 hormone), despite having normal TSH levels, have shown T3 levels which are even lower than T4. In order to bring T3 levels back to the normal range, higher doses of levothyroxine are required.
Moreover, according to The American Thyroid Association normal levels of TSH, T3 and T4 may not be a fair indication of euthyroidism, or normality. The body may attempt to reduce metabolic expenditure- a process controlled by the thyroid hormones, when subjected to stressful situations such as chronic disease, chronic stress, chronic fatigue or fibromyalgia, old age, leptin resistance, diabetes, depression and/or obesity. When metabolic expenditure is reduced, activity of the enzyme responsible for converting T4 into T3 is slowed, resulting in «low T3 syndrome» or “euthyroid sick syndrome”.
While the conventional approach to Hashimoto’s disease takes into consideration the hormonal deficit caused by glandular destruction it does not address the multiple mechanisms involved in the disease’s origin and development. A multidisciplinary approach, on the other hand, considers the various related factors and the resulting multisystemic consequences.
Some factors taken into consideration in a multidisciplinary approach to Hashimoto’s disease are: thyroid hormone therapy, nutrition, supplements, heavy metals, stress and emotional management, and physical activity.
1 Thyroid hormone therapy
The usual treatment for Hashimoto’s disease is thyroid hormone replacement therapy with levothyroxine (synthetic T4). As Hashimoto’s disease involves a deficiency of thyroid hormones T3 and T4, a combination of the two thyroid hormones could prove more promising. This aspect, however, is under study due to the difficulties of therapeutic compliance and the difficulty of controlling the fluctuating serum levels of thyroid hormones.
2 Nutrition
Nutrition has a fundamental effect on Hashimoto’s disease due to the cellular inflammation and alterations in the intestinal barrier that occur as the disease progresses. An anti-inflammatory diet is recommended, including organic and non-processed foods such as fruit with low glycemic index, vegetables, and small- or medium-sized oily fish. Forgo processed foods, trans fats and any grains that may contain gluten. «Gluten-free» is often a poor substitute, though quinoa, rice, buckwheat, teff, or amaranth are all good substitutes for those items which usually contain gluten.
Soy, too, should be avoided as it reduces thyroid hormone bioavailability, inhibits the enzyme that synthesizes thyroid hormones, and prevents the body’s ability to absorb iodine.
Isothiocyanates are phytochemicals present in crucifers (cabbage, broccoli, brussels sprouts, cabbage, cauliflower) which lower the thyroid’s ability to absorb iodine. When suffering from iodine deficiency, or when consumed raw, such foods can be especially damaging.
3 Supplements
Supplements can be a good option to treat or reduce symptoms associated with Hashimoto’s disease.
- Selenomethionine (Selenium): facilitates the synthesis of thyroid hormones, the reduction of autoantibodies and improves the quality of life of people suffering from Hashimoto’s disease.
Iodine: the diet would be sufficient to provide the necessary amount of iodine.
- Vitamin D
- Lipoic acid: may have a positive effect on the peripheral metabolism of thyroid hormones, although one study suggests that lipoic acid reduces the effect of levothyroxine.
- Digestive enzymes: digestive enzyme deficiencies can cause digestion problems that can contribute to nutrient malabsorption, food intolerance, bacterial overgrowth, and autoimmune diseases. Supplementation with digestive enzymes may help correct enzyme deficiencies and improve conditions related to altered digestion and absorption, although no direct link to Hashimoto’s disease has been established.
- Nutrients to correct permeability of the intestines: essential fatty acids (omega 3 and omega 6), l-glutamine, n-acetyl-glucosamine (NAG), lion’s mane.
- Antioxidants: quercetin, ginkgo biloba, vitamins C and E, N-acetyl-cysteine: reduce inflammatory parameters and contribute to the care of the intestinal mucosa.
- Prebiotics (galactooligosaccharides, fructooligosaccharides): present stabilizing effects to the intestinal barrier and modulate the gut microbiota.
- Probiotics: microorganisms administered orally to help maintain or restore the beneficial intestinal microflora and prevent or treat gastrointestinal disorders and related systemic conditions.
- Zeolites: Zeolites are crystalline compounds with microporous structures that may act in the intestine as adsorbents, detergents, or anti-diarrheal agents, among other effects. Zeolite supplementation has shown a positive effect on intestinal mucosal integrity and mild anti-inflammatory effects.
- Mycotherapy: Medicinal mushroom extracts such as Reishi, Shiitake, Maitake or Chaga have been recognized for their anti-inflammatory and immune system modulating properties. They have also shown activity against EBV, CMV and the herpes virus
- Bovine colostrum: In addition to its effect on the intestinal mucosa, bovine colostrum has a direct effect on immunity. Recent studies suggest that the components of colostrum, immunoglobulin and growth factor physically benefit the individual as well as treatment of autoimmune disorders.
Others: Vitamin A, B12, Zinc and Iron.
4 Heavy metals
Exposure to heavy metals, especially mercury, should be avoided as much as possible. Large fish such as tuna, shark and swordfish are best avoided. The safest fish to consume are anchovies, cod, mackerel, sole, hake and sardines. Surprisingly, the most significant source of inorganic mercury in our diets is dental amalgam, a liquid mercury and metal alloy mixture used to fill cavities caused by tooth decay. Conditions must be safe, therefore, when removing these amalgams, with the application of a heavy metal chelating treatment.
If heavy metal poisoning is confirmed, treatments are available:
There are a number of substances, such as Chlorella pyrenoids, which can prevent heavy metal absorption.
Some chelating substances exhibit a high degree of affinity for metal: ethylenediaminetetraacetic acid (EDTA), DMSA and DMPS.
Certain substances have detoxifying and antioxidant potential. Some examples are glutathione (with specific effects on mercury toxicity), alpha-lipoic acid, N-acetylcysteine and folic acid.
Induced sweating: i.e. saunas. In addition to its many positive health effects, saunas are also effective in removing heavy metals and other toxins, such as bisphenol A and polychlorinated biphenyls.
5 Stress and emotional management
Although the direct mechanism by which stress impacts Hashimoto’s disease is unclear, there are clear indications that it alters processes linked to immunity and inflammation.
Several studies show the efficacy of meditation and yoga for improving stress management. Although there are no specific studies on patients with Hashimoto’s disease, there have been reviews on two autoimmune diseases of a similar nature: multiple sclerosis and rheumatoid arthritis. These show consistent evidence for the use of such techniques for reducing stress and the subsequent impact on autoimmune disease.
Nutritional supplements have also been used to promote stress management. Some examples are rhodiola rosa- which positively affects physical and mental performance); vitamin B, magnesium and fatty acids, and withania somnifera (aswagandha)- which attenuates oxidative damage, reduces anxiety, and has been shown to reduce TSH in people suffering from subclinical hypothyroidism.
6 Physical activity
Physical activity has been shown to achieve an elevation of lymphocyte-regulating cells, and promotes the release of IL-6 muscles (which induce inflammatory response). There are no studies between physical activity and Hashimoto’s disease. In other autoimmune diseases, however, the incidence of rheumatoid arthritis, psoriasis, multiple sclerosis and inflammatory bowel disease is higher among those who engage in lower levels of physical activity. Research also exists on the effect that physical activity has on thyroid function and has shown that regular exercise has a positive effect on people with hypothyroidism, improving their thyroid hormone profile.
The treatment of Hashimoto’s disease, in conclusion, must include a multidisciplinary approach that is able to address the different factors implicated in the source of the disease, the means by which it was developed, and the implications the disease has on the body as a whole. Diet, nutritional supplements, physical activity and stress management have all brought about positive results not only in analytical testing, but also in the quality of life of those who suffer from it.
Todos los contenidos ofrecidos por El camino de Lola, tales como artículos, podcasts y videos, tienen naturaleza meramente informativa y en ningún caso constituyen servicio médico o sanitario de ningún tipo ni sustituyen la consulta con un médico especialista, por lo que no deben ser aplicados sin la aprobación previa y supervisión de un médico o profesional de la salud especializado.
¿Qué te ha parecido este artículo?
Déjanos un comentario. Gracias.